Achieve Life Sciences Announced Granting of New Hire Inducement Awards
SEATTLE and VANCOUVER, British Columbia, Jan. 30, 2026 (GLOBE NEWSWIRE) — Achieve Life Sciences, Inc. (Nasdaq: ACH
Read More

We are a late-stage specialty pharmaceutical company dedicated to tackling the global nicotine dependence epidemic by advancing cytisinicline.
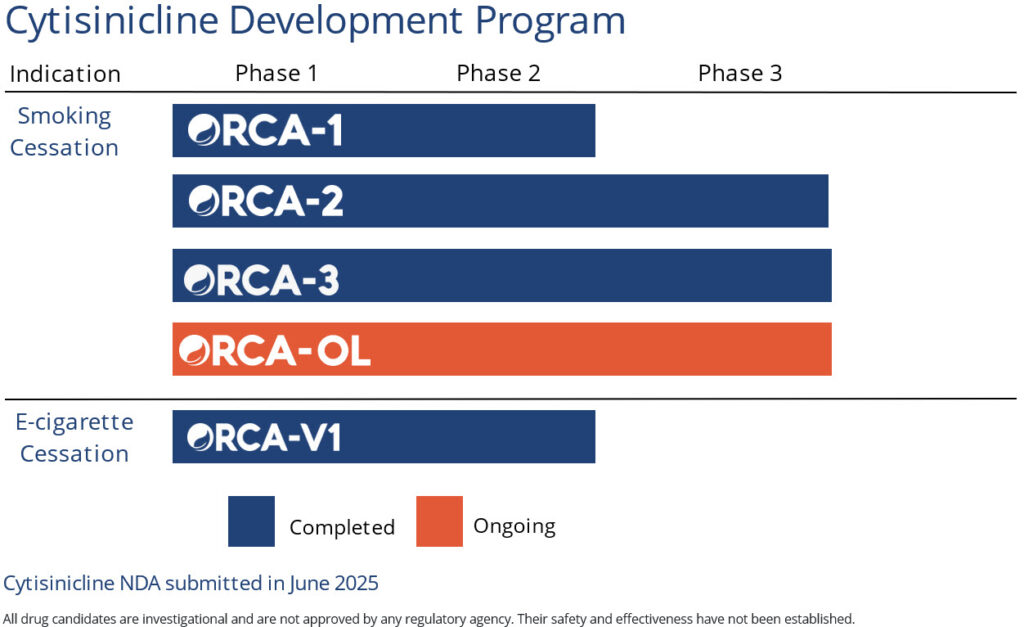
In September 2025, our New Drug Application, submitted to the FDA in June 2025, was accepted for review. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) date of June 20, 2026 for cytisinicline as a treatment of nicotine dependence for smoking cessation in adults, based on two successfully completed Phase 3 studies and its fully enrolled open-label safety study.
If approved, cytisinicline has the potential to become the first new FDA-approved treatment option in nearly 20 years.
Smokers in the US 1
E-Cigs in the US 2

The harms associated with smoking are well established, increasing the risk of COPD, cardiovascular disease, cancer, and other serious conditions.
While over half attempt to quit smoking each year, fewer than 10% succeed.1 The toll is staggering – lost lives and over $600 billion in annual smoking-related healthcare costs.14
A more effective treatment is critical to addressing this widespread public health crisis.
Worldwide Deaths Annually4
Americans Live with Smoking- Related Disease5

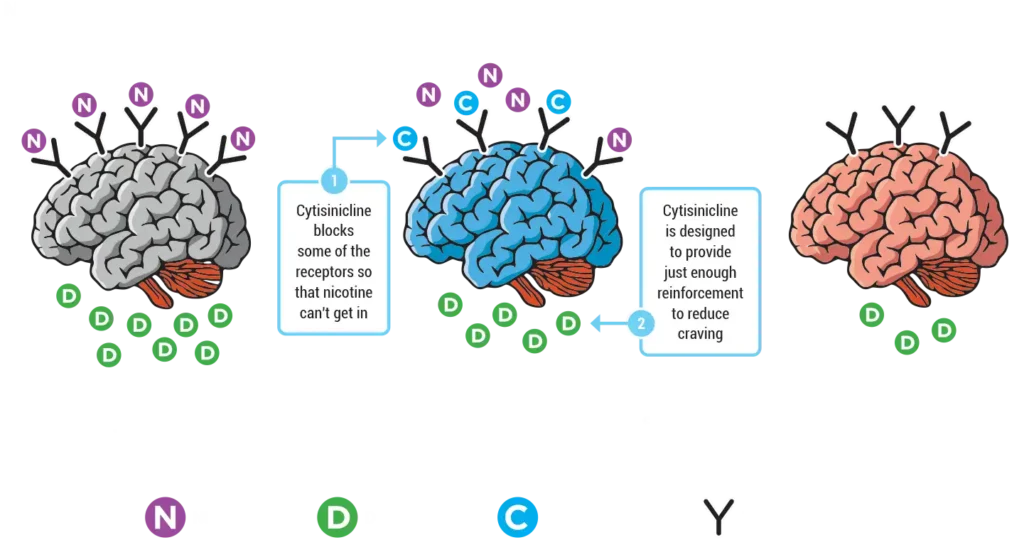
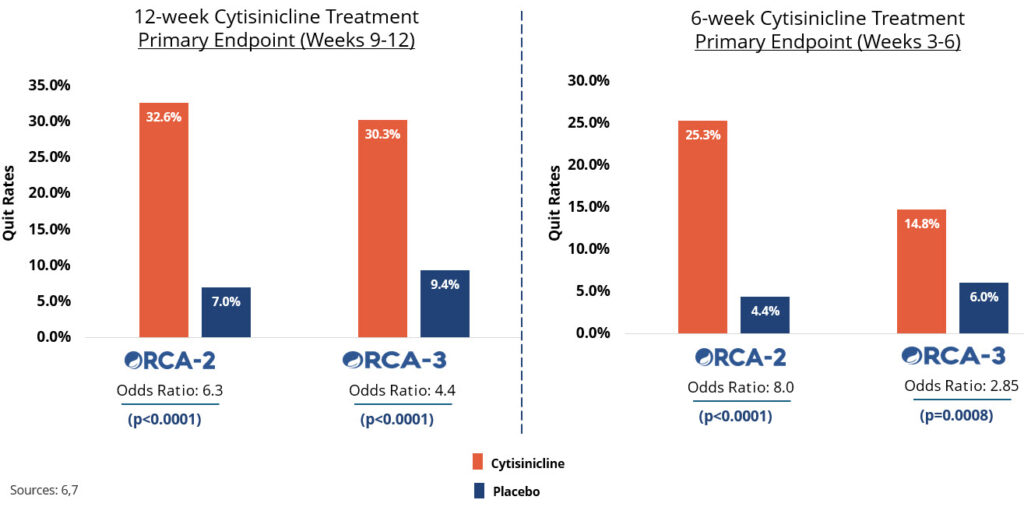
In two recent trials, cytisinicline has been shown to effectively help even highly dependent smokers stop smoking.6,7 Cytisinicline binds to the same receptors as nicotine to reduce both withdrawal symptoms and satisfaction associated with smoking.8 And since it doesn’t bind as strongly to certain receptors, it is believed to have less potential to cause side effects — including nausea and vomiting.


And we’re looking for others, like us, who believe we can bring an end to this fight. Will you join us?