|
|
A Second Multicenter, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Trial of Cytisinicline in Adult Smokers
 Topline Data Results Reported in Q2′2023
Topline Data Results Reported in Q2′2023
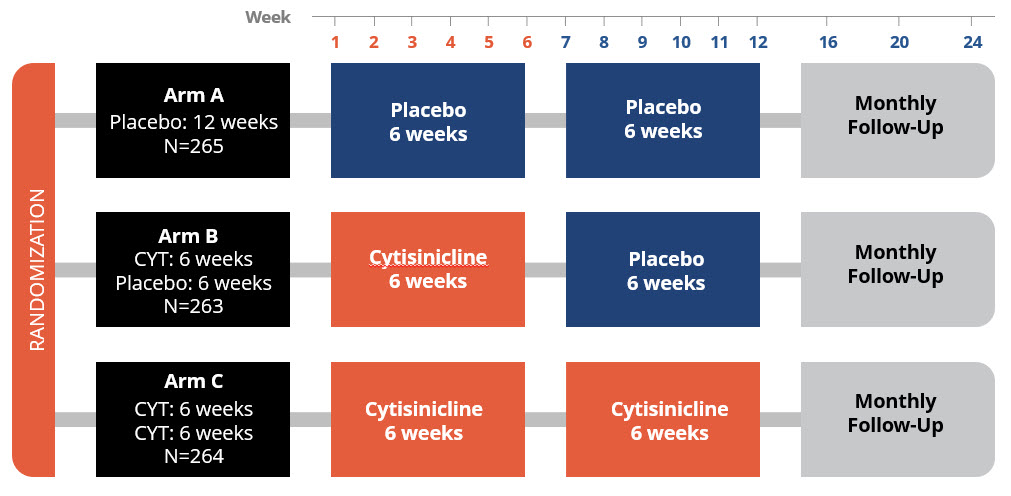
ORCA-3 was designed to evaluate the efficacy and safety of 3mg cytisinicline dosed 3 times daily for a period of 6 weeks or 12 weeks compared to placebo. ORCA-3 randomized 792 adult smokers at 20 clinical trial sites in the United States. All participants received standard behavioral support for the duration of the trial.
DATA RESULTS
The primary endpoint for ORCA-3 was biochemically verified smoking cessation measured during the last 4 weeks of treatment. Subjects were monitored for smoking cessation for 24 weeks post randomization.
Both the 6- and 12-week cytisinicline treatment durations demonstrated statistically significant smoking cessation on both the primary and secondary efficacy analyses compared to placebo.
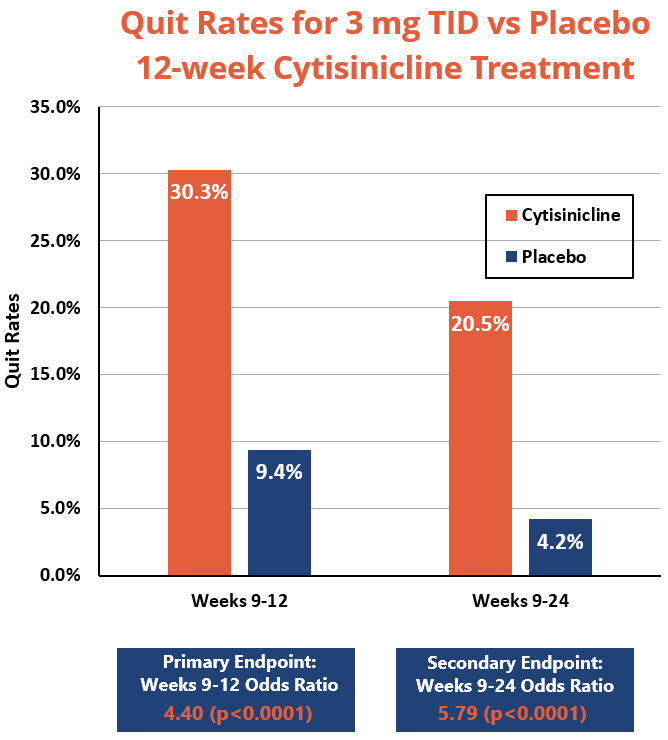
- Primary Endpoint: Subjects who received 12 weeks of cytisinicline treatment had 4.4 times higher odds, or likelihood, to have quit smoking during the last 4 weeks of treatment compared to subjects who received placebo (p<0.0001). The smoking cessation rate during weeks 9 through 12 was 30.3% for cytisinicline compared to 9.4% for placebo.
- Secondary Endpoint: The continuous smoking cessation rate from week 9 to week 24 was 20.5% for the 12-week cytisinicline arm compared to 4.2% for placebo, with an odds ratio of 5.79 (p<0.0001).
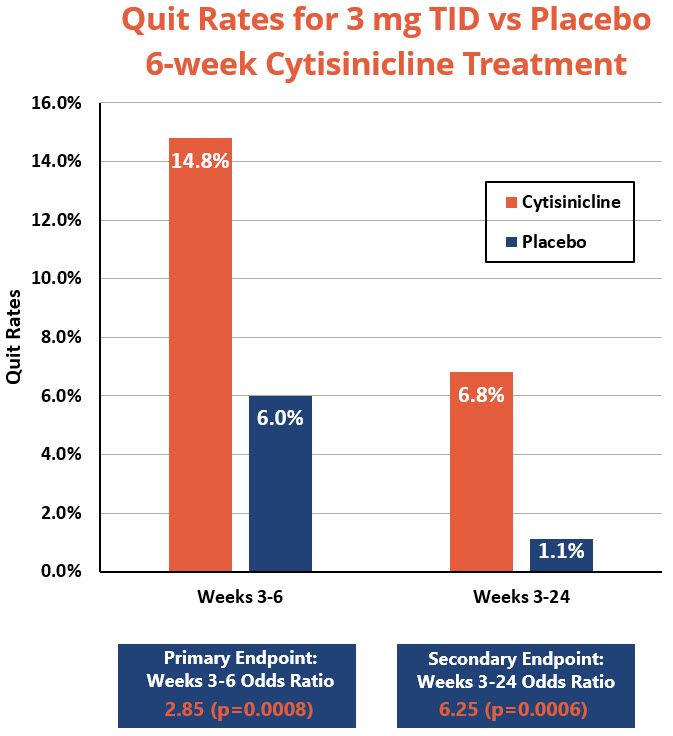
- Primary Endpoint: Subjects who received 6 weeks of cytisinicline treatment had 2.85 times higher odds, or likelihood, to have quit smoking during the last 4 weeks of treatment compared to subjects who received placebo (p=0.0008). The smoking cessation rate during weeks 3 through 6 was 14.8% for cytisinicline compared to 6% for placebo.
- Secondary Endpoint: The continuous smoking cessation rate from week 3 to week 24 was 6.8% for the 6-week cytisinicline arm compared to 1.1% for placebo, with an odds ratio of 6.25 (p=0.0006).
SAFETY PROFILE
- Well-tolerated with low rates of adverse events (AEs)
- No treatment-related serious adverse events reported
Most commonly reported AEs (>5% Overall)
| Adverse Events | Placebo (N=262) | 6-week CYT (N=263) | 12-week CYT (N=260) |
|---|---|---|---|
| At least 1 TEAE | 164 (62.6%) | 170 (64.6%) | 168 (64.5%) |
| Insomnia | 20 (7.6%) | 29 (11.0%) | 31 (11.9%) |
| Abnormal Dreams | 15 (5.7%) | 24 (9.1%) | 20 (7.7%) |
| Nausea | 19 (7.3%) | 25 (9.5%) | 18 (6.9%) |
| Headache | 16 (6.1%) | 20 (7.6%) | 22 (8.5%) |
Low rates of AEs compare favorably to currently approved smoking cessation products.
Design
The ORCA-3 trial mirrored the Phase 3 ORCA-2 trial and was designed to obtain additional safety and efficacy data.
- Placebo-controlled & double-blind
- Three treatment arms: Placebo vs 6 or 12 weeks cytisinicline
- Cytisinicline treatment for 6 or 12 weeks
- Does longer treatment lead to better efficacy?
- Does continued treatment prevent early relapses?
- Primary Endpoint: four-week continuous abstinence ON treatment
- Follow up to six months