Join us in redefining the future of nicotine dependence
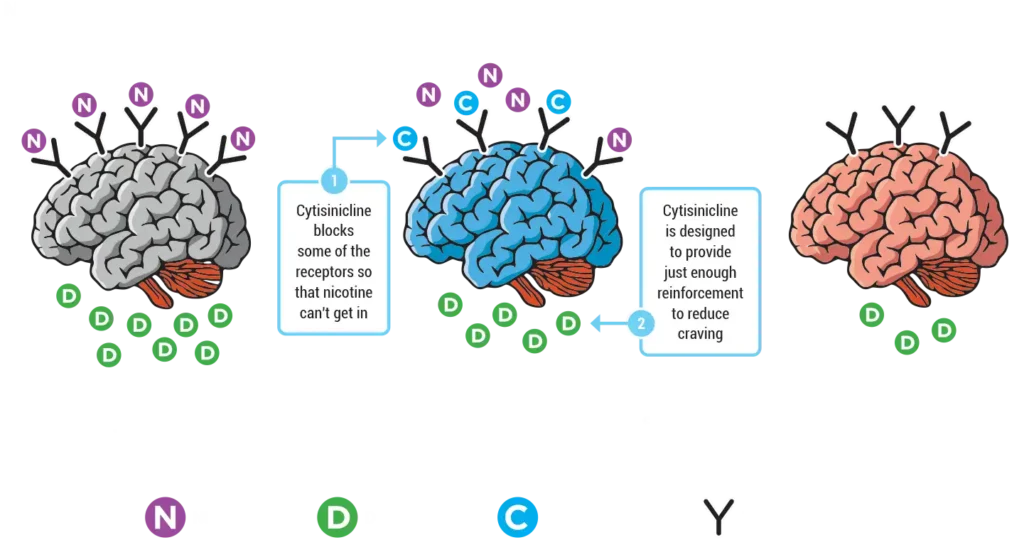
We are a late-stage specialty pharmaceutical company dedicated to tackling the global nicotine dependence epidemic by advancing cytisinicline.
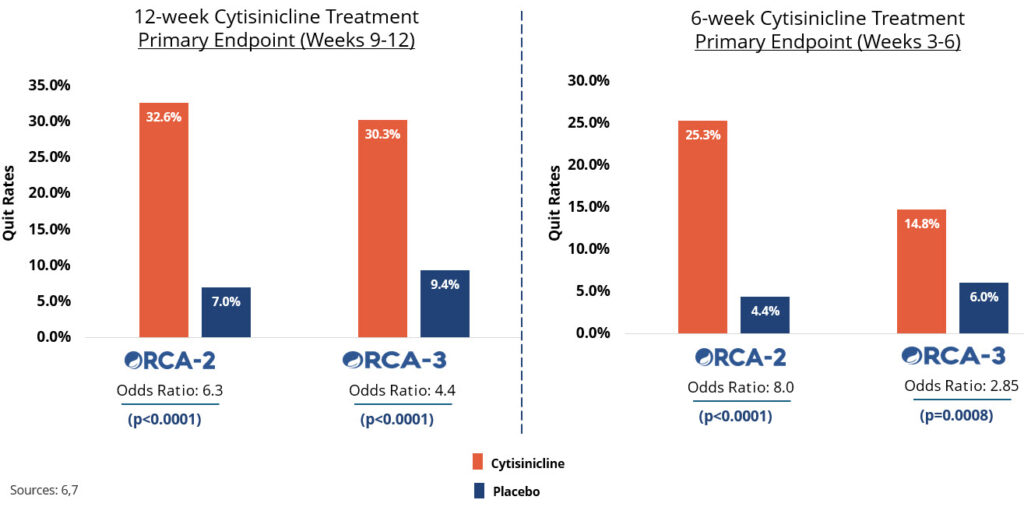
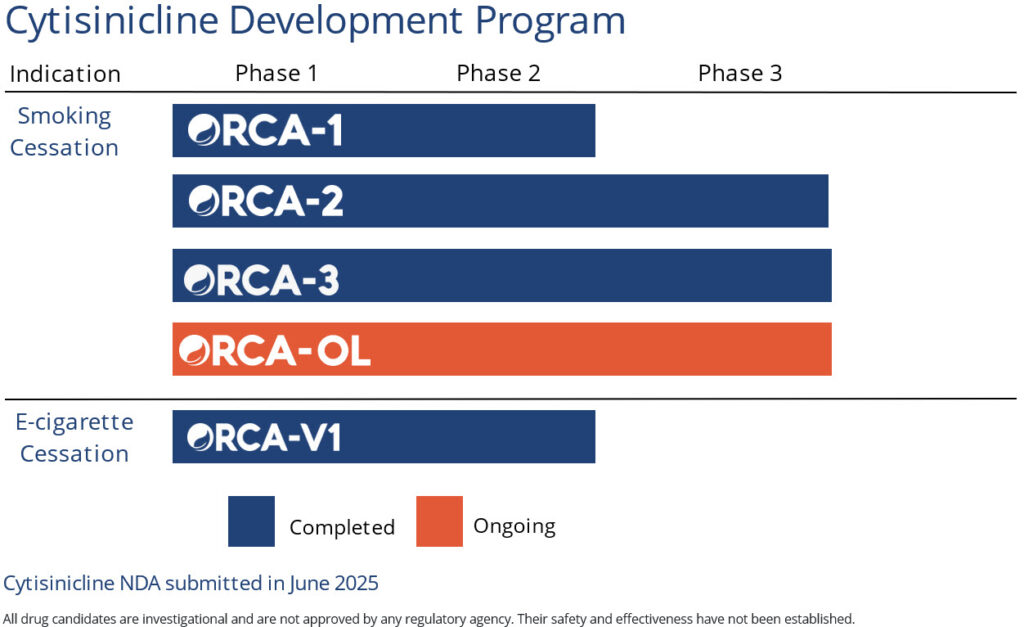
In September 2025, our New Drug Application, submitted to the FDA in June 2025, was accepted for review. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) date of June 20, 2026 for cytisinicline as a treatment of nicotine dependence for smoking cessation in adults, based on two successfully completed Phase 3 studies and its fully enrolled open-label safety study.
If approved, cytisinicline has the potential to become the first new FDA-approved treatment option in nearly 20 years.