|
|
A Multicenter, Double-Blind, Randomized, Placebo-Controlled Phase 2b Trial of Cytisinicline in Adult Smokers
 Oxford Academic: Nicotine & Tobacco Research | Vol 23
Oxford Academic: Nicotine & Tobacco Research | Vol 23
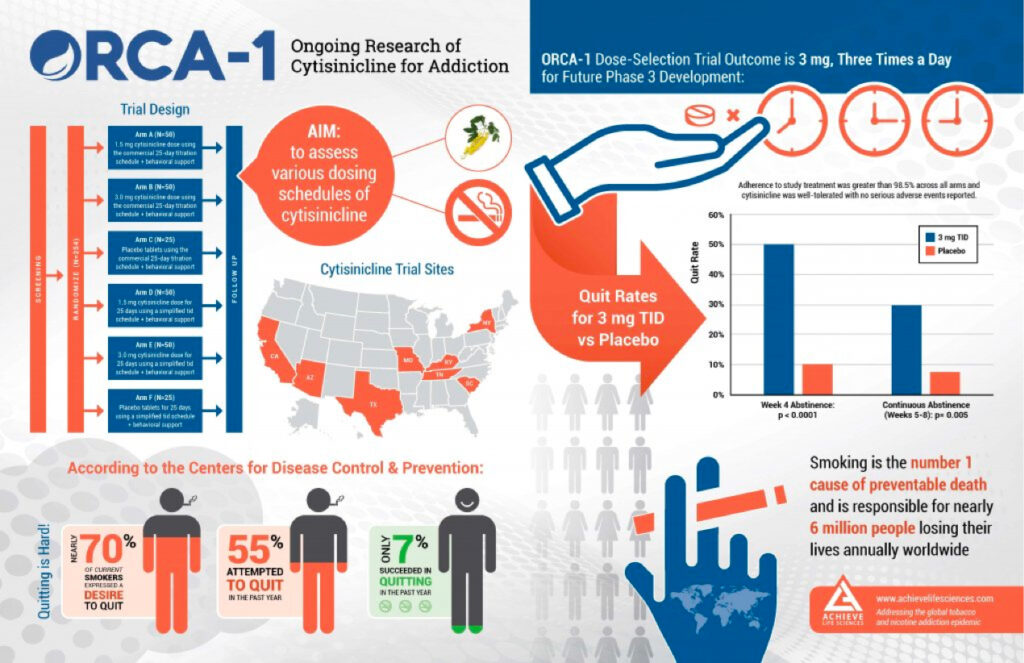
This Phase 2b trial was conducted in the US to assess various dosing schedules of cytisinicline in approximately 254 smokers. It evaluated efficacy based on the overall reduction in the number of cigarettes smoked during the various study treatment doses and schedules. It also evaluated compliance and safety profiles compared to the respective placebo arms. This dose-finding study resulted in identification of the 3mg, three-times-daily dosing regimen to be used in future cytisinicline Phase 3 trials.
Data Results
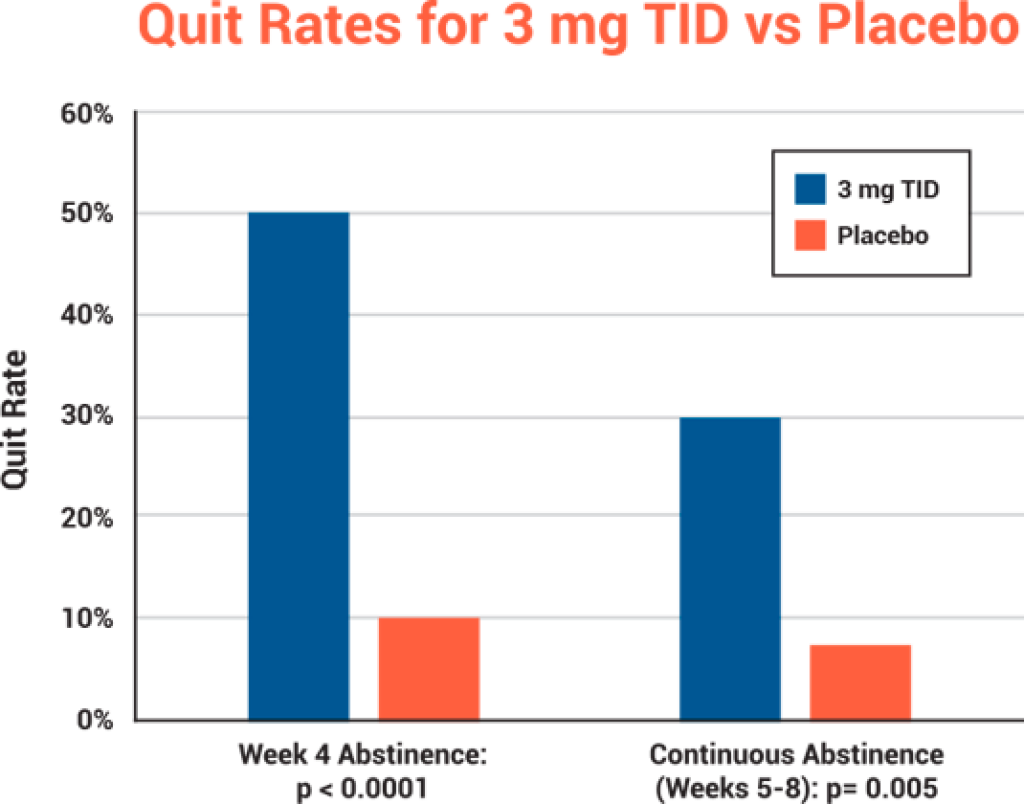
- Statistically significant quit rates demonstrated at both end of treatment and weeks 5 through 8 (the FDA approvable endpoint)
- Adherence to study treatment was 98% in the 3mg TID arm
- Cytisinicline was well-tolerated with no serious adverse events reported
- 3mg dose with TID administration selected to move forward to Phase 3 development
| Characteristic | 3mg CYT (N=50) |
Placebo (N=51) |
P Value |
|---|---|---|---|
| Reduction in Expired CO1 | 80% | 38% | p = 0.003 |
| Four-Week Abstinence2 | 50% | 10% | p < 0.001 |
| Continuous Abstinence (Weeks 5-8)3 | 30% | 8% | p = 0.005 |
|
|||
Safety
Most commonly reported (>5%) side effects from ORCA-1:
| Adverse Event | 3mg TID (N=50) |
Pooled Cytisinicline (N=203) |
Placebo (N=51) |
|---|---|---|---|
| At least 1 AE | 46% | 46% | 47% |
| Upper Respiratory Tract Infections | 6% | 6% | 14% |
| Nausea | 6% | 6% | 10% |
| Abnormal Dreams | 6% | 9% | 2% |
| Insomnia | 6% | 7% | 2% |
| Constipation | 6% | 2% | 2% |
| Headache | 2% | 5% | 2% |
Results:
- Cytisinicline was well-tolerated across all treatment groups
- Overall low incidence of adverse events
- No serious or severe adverse events reported
Study Design
This six-arm, multi-center, double-blind, randomized, placebo-controlled, Phase 2b study was conducted in the US. The study was double-blinded to dose but not to the administration schedule. Subjects provided a quit date 5-7 days from randomization. Study treatment started the day after randomization such that study treatment was initiated prior to the quit date.
Demographics were similar between schedules and for treatment arms in gender, age, years of prior smoking, and number of previous quit attempts. At baseline, subjects in the study reported smoking a median of 20 cigarettes per day. ORCA-1 was initiated in October 2018 and enrolled 254 smokers at eight centers across the United States.