|
|
A Multicenter, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Trial of Cytisinicline in Adult Smokers
 Journal of the American Medical Association | July 2023
Journal of the American Medical Association | July 2023
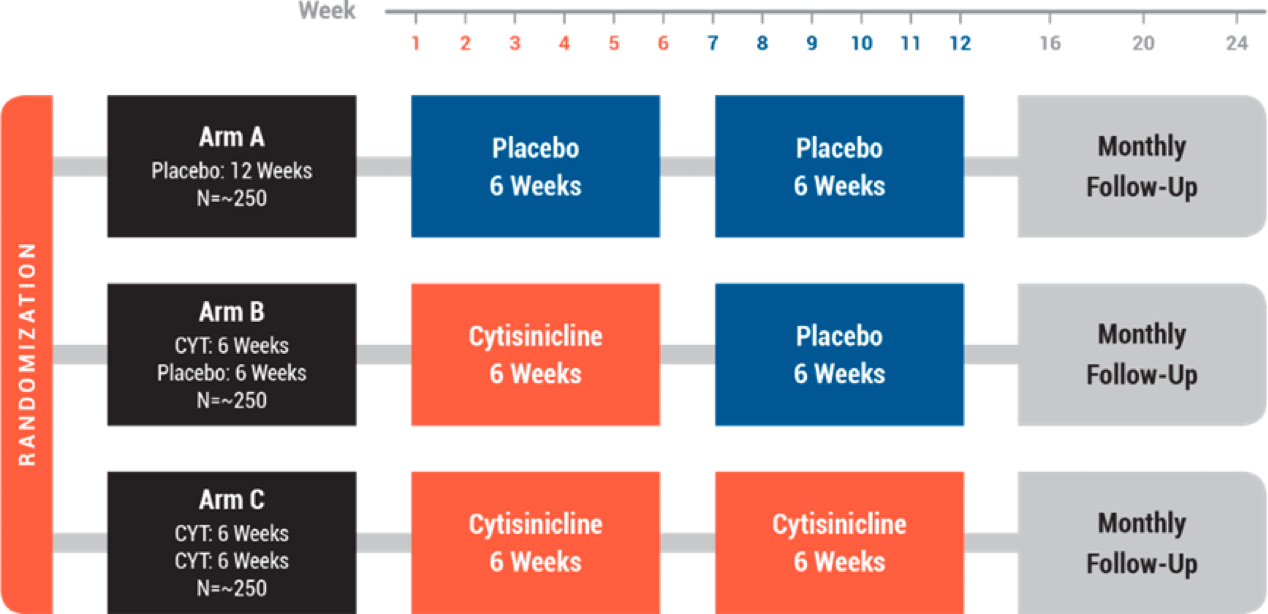
ORCA-2 was designed to evaluate the efficacy and safety of 3mg cytisinicline dosed 3 times daily for a period of 6-weeks or 12-weeks compared to placebo in 810 adult smokers (randomized 1:1:1). Subjects were monitored for smoking abstinence for 24 weeks post randomization and received standard behavioral support for the duration of the trial.
DATA RESULTS
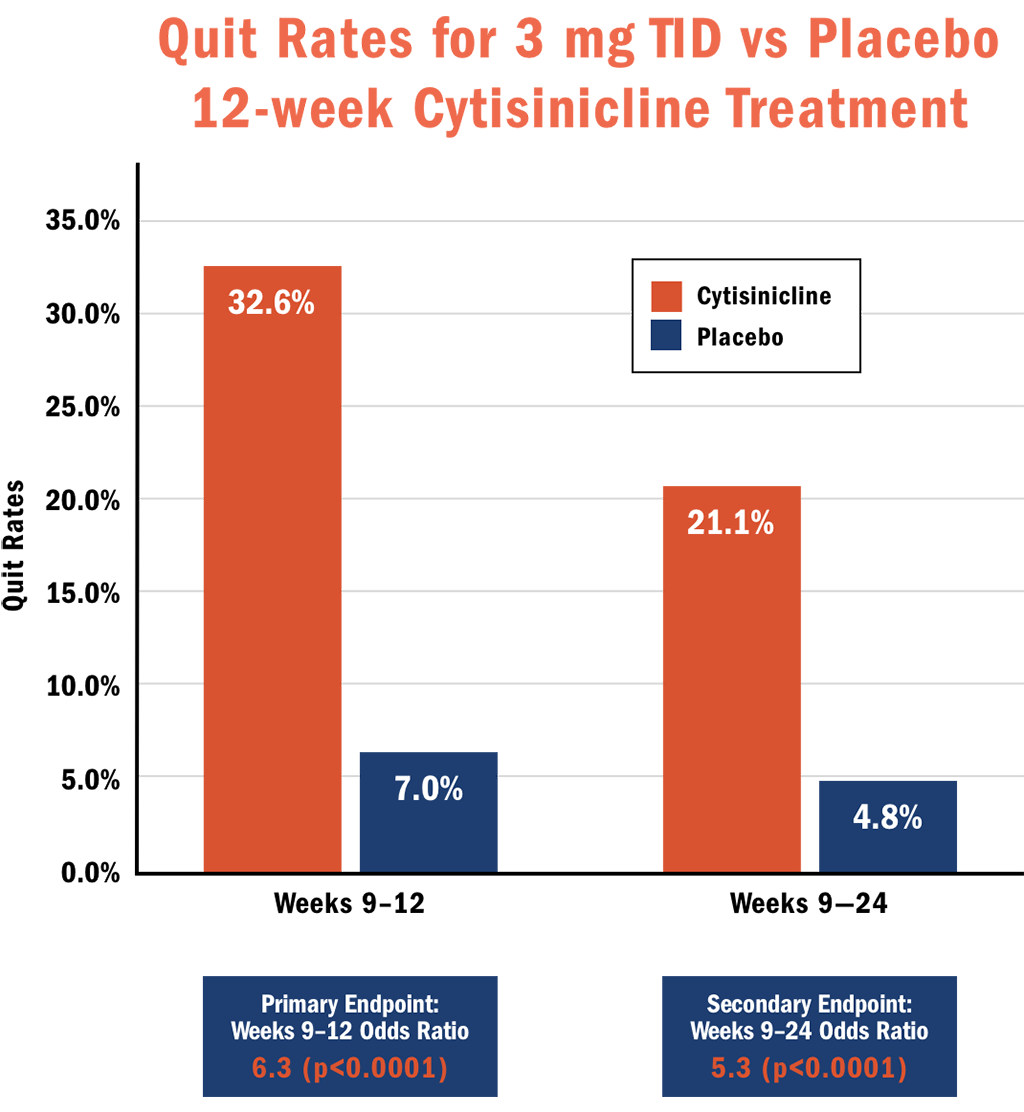
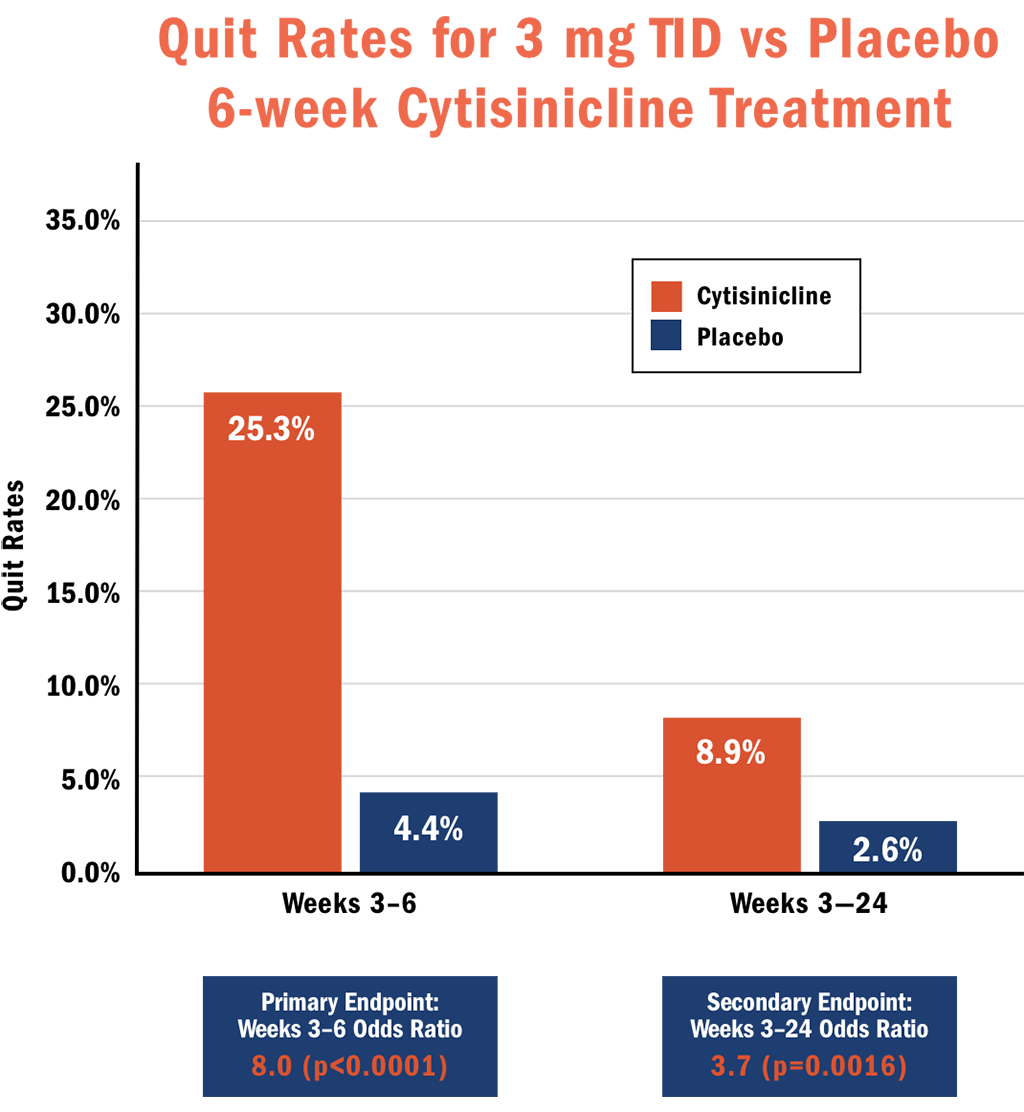
The primary endpoints for ORCA-2 were biochemically verified continuous abstinence measured during the last 4 weeks of treatment. Both the 6- and 12-week cytisinicline treatments demonstrated significantly better quit rates than placebo with odds ratios of 8.0 and 6.3, respectively.
- Subjects who received 12 weeks of cytisinicline treatment had 6.3 times higher odds, or likelihood, to have quit smoking during the last 4 weeks of treatment compared to subjects who received placebo (p<0.0001). The abstinence rate during weeks 9-12 was 32.6% for cytisinicline compared to 7.0% for placebo.
- Subjects who received 6 weeks of cytisinicline treatment had 8 times higher odds, or likelihood, to have quit smoking during the last 4 weeks of treatment compared to subjects who received placebo (p<0.0001). The abstinence rate during weeks 3-6 was 25.3% for cytisinicline compared to 4.4% for placebo.
SAFETY PROFILE
- Well-tolerated with single digit rates of adverse events (AEs)
- No treatment-related serious adverse events reported
Most commonly reported AEs (>5% Overall)
| Adverse Events | Placebo (N=270) |
6-week CYT (N=269) |
12-week CYT (N=270) |
|---|---|---|---|
| At least 1 TEAE | 166 (61.5%) | 172 (63.9%) | 184 (68.1%) |
| Insomnia | 13 (4.8%) | 23 (8.6%) | 26 (9.6%) |
| Abnormal Dreams | 8 (3.0%) | 22 (8.2%) | 21 (7.8%) |
| Headaches | 22 (8.1%) | 18 (6.7%) | 21 (7.8%) |
| Nausea | 20 (7.4%) | 16 (5.9%) | 15 (5.6%) |
Low rates of AEs compare favorably to currently approved smoking cessation products.
Design
The ORCA-2 Trial evaluated the safety and efficacy of 3mg cytisinicline dosed three times daily for 6 and 12 weeks compared to placebo.
- Placebo-controlled & double-blind
- Three treatment arms: Placebo vs 6 or 12 weeks cytisinicline
- Cytisinicline treatment for 6 or 12 weeks
- Does longer treatment lead to better efficacy?
- Does continued treatment prevent early relapses?
- Primary Endpoint: four-week continuous abstinence ON treatment
- Follow up to six months